Home
|
Products
|
9789356962026
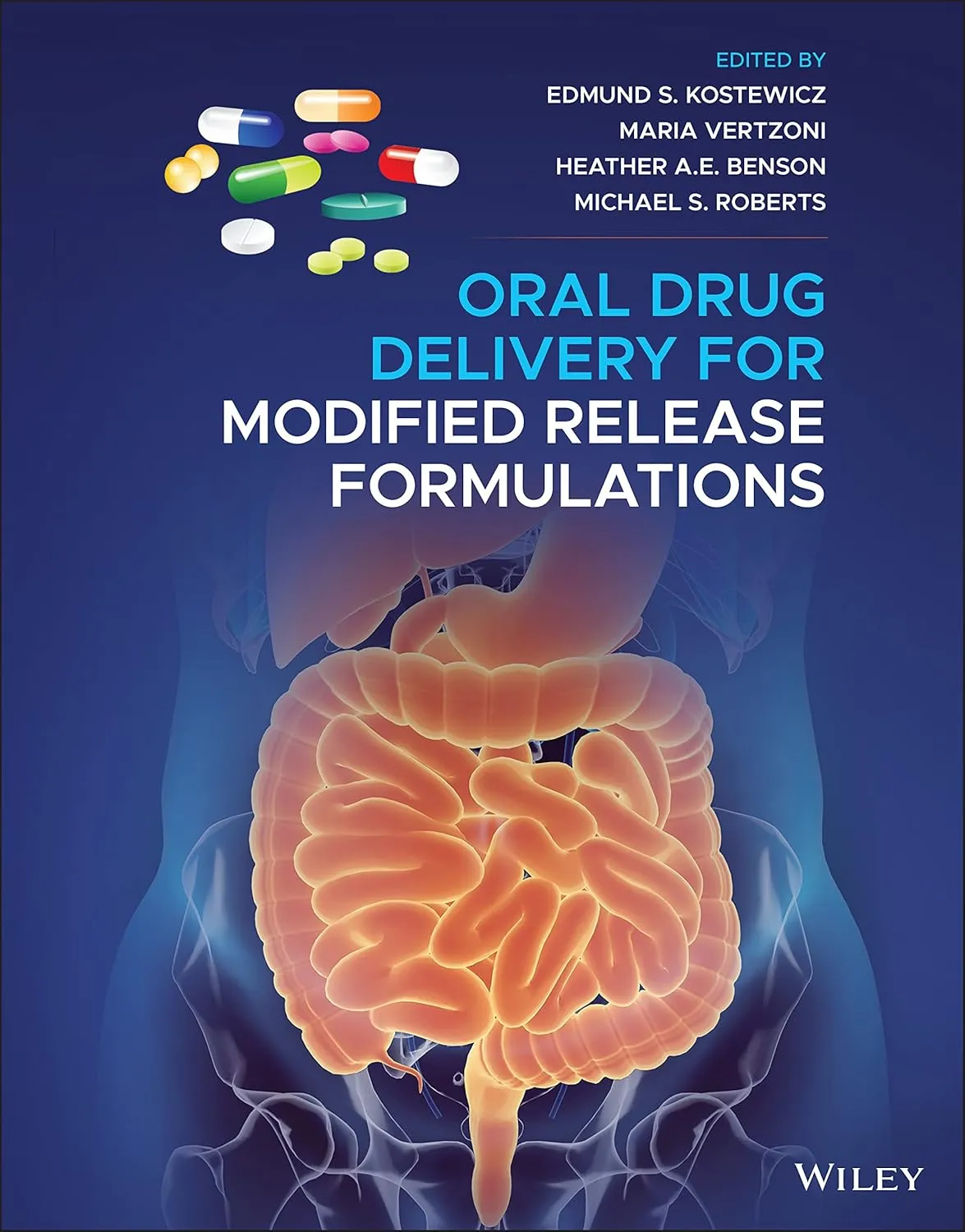
Oral Drug Delivery For Modified Release Formulations | Hardcover
by Kostewicz E.S.
Highlights
9781119772699
ISBN
Kostewicz E.S.
Author

496
Pages

215 gm
Weight

English
Language

2022
Year

1st Edition
Edition

Hardcover
Binding
₹18717
₹20797
ORAL DRUG DELIVERY FOR MODIFIED RELEASE FORMULATIONSProvides pharmaceutical development scientists with a detailed reference guide for the development of MR formulationsOral Drug Delivery for Modified Release Formulations is an up-to-date review of the key aspects of oral absorption from modified-release (MR) dosage forms. This edited volume provides in-depth coverage of the physiological factors that influence drug release and of the design and evaluation of MR formulations. Divided into three sections, the book begins by describing the gastrointestinal tract (GIT) and detailing the conditions and absorption processes occurring in the GIT that determine a formulations oral bioavailability. The second section explores the design of modified release formulations, covering early drug substance testing, the biopharmaceutics classification system, an array of formulation technologies that can be used for MR dosage forms, and more. The final section focuses on in vitro, in silico, and in vivo evaluation and regulatory considerations for MR formulations. Topics include biorelevant dissolution testing, preclinical evaluation, and physiologically-based pharmacokinetic modelling (PBPK) of in vivo behaviour. Featuring contributions from leading researchers with expertise in the different aspects of MR formulations, this volume: Provides authoritative coverage of physiology, physicochemical determinants, and in-vitro in-vivo correlation (IVIVC)Explains the different types of MR formulations and defines the key terms used in the fieldDiscusses the present status of MR technologies and identifies current gaps in researchIncludes a summary of regulatory guidelines from both the US and the EUShares industrial experiences and perspectives on the evaluation of MR dosage formulationsOral Drug Delivery for Modified Release Formulations is an invaluable reference and guide for researchers, industrial scientists, and graduate students in general areas of drug delivery including pharmaceutics, pharmaceutical sciences, biomedical engineering, polymer and materials science, and chemical and biochemical engineering.
Online store of medical books
Discover a comprehensive range of medical books at our online store. From anatomy and physiology to the latest clinical guidelines, we've got you covered.
Trusted by students, educators, and healthcare professionals worldwide. Browse top publishers and expert-authored titles in every medical specialty. Enjoy fast shipping, secure payments, and easy returns. Your one-stop destination for quality medical knowledge at your fingertips.
Whether you're preparing for exams or expanding your clinical expertise, our curated collection ensures you have the right resources at hand. Dive into detailed illustrations, case studies, and up-to-date research that enhance your understanding and practical skills.
We regularly update our inventory to include the latest editions and newly released titles, helping you stay current in the ever-evolving medical field. Our advanced search and filtering tools make finding the perfect book quick and hassle-free.
Join our community of lifelong learners and medical enthusiasts. Sign up for exclusive discounts, early access to new arrivals, and personalized book recommendations tailored to your professional interests.
